The Unbearable Lightness of Hydrogen

The problem of hydrogen storage
HYDROGEN has long been considered a transformative technology for the energy sector, as it fulfils the main requirements of a clean and sustainable energy vector. It is abundant in the Universe and on Earth, has the highest energy density on a mass basis of any chemical fuel, and can be easily converted between different forms of energy. For all of these reasons, hydrogen is considered one of the best options for decarbonising the transport sector, which is still almost completely reliant on fossil fuels.
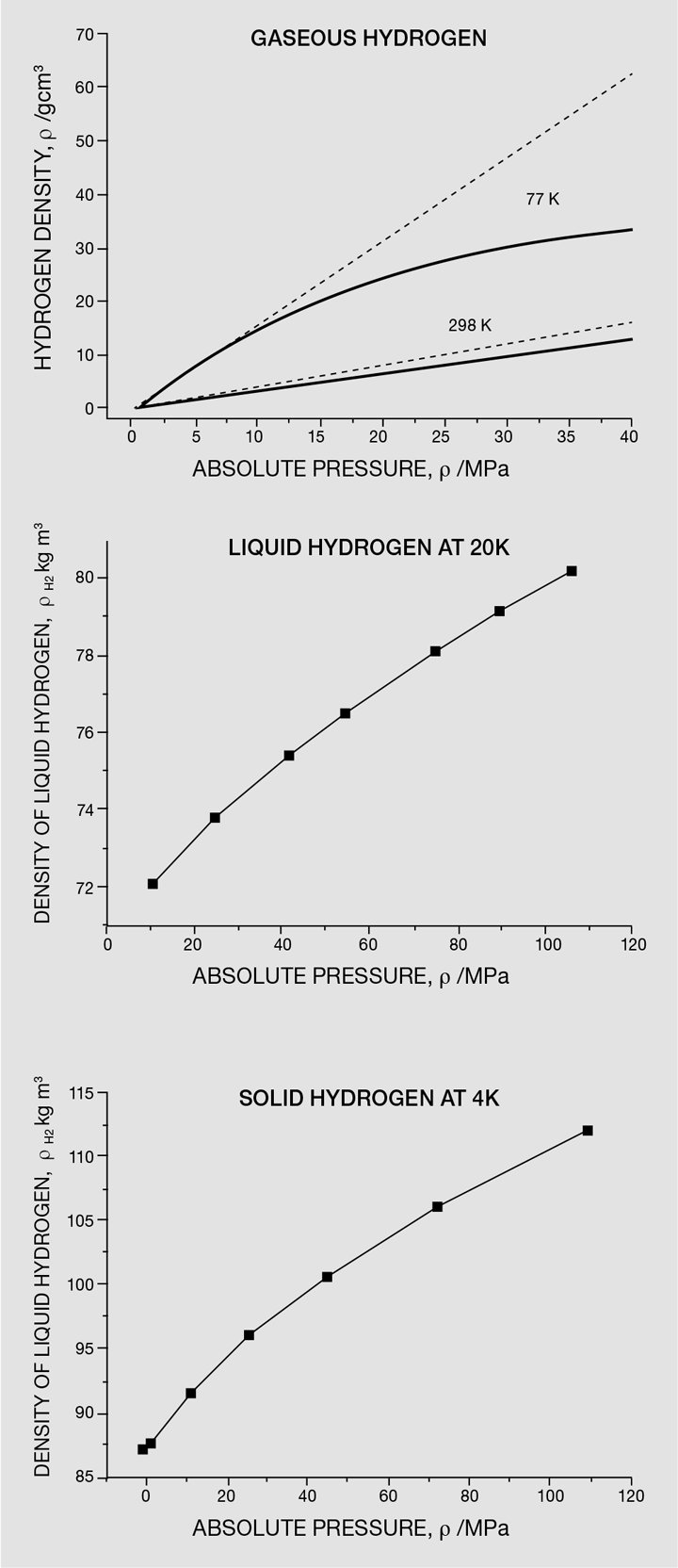
A number of technical issues still preclude the widespread use of hydrogen as an energy vector, at the top of which is how to store hydrogen in an affordable, sustainable and safe manner. This difficulty comes from the physical properties of hydrogen – hydrogen is the first element in the periodic table and it is the lightest. Free hydrogen exists as a diatomic molecule (H2) and has a density at standard temperature and pressure (0 °C and 0.1 MPa) of around 0.09 kg/m3 [1], which is significantly less than air (1.3 kg/m3). Its normal boiling and melting points are around -253 °C and -259 °C, respectively, and even as a liquid or a solid, it has extremely low densities. Liquid hydrogen at -253 °C and 0.1 MPa has a density of 71 kg/m3 [2] and solid hydrogen at -259 °C and 0.1 MPa has a density of 88 kg/m3 [3]. Hydrogen has some peculiar properties, including the fact that it can be compressed as a liquid and as a solid. Figure 1 shows the density of hydrogen in the gaseous, liquid and solid state at increasing pressures.
Hydrogen has a very large energy density on a mass basis, but the challenge is to improve on its volumetric energy density. To achieve this, a number of different technologies have been proposed, with the main goal being to design a hydrogen storage system that is affordable, sustainable, safe, and one in which hydrogen can be easily and quickly charged and discharged. Research in this area has been driven by targets created by the US Department of Energy for hydrogen storage systems in automotive applications [4]. These targets look at a number of practical requirements, including gravimetric and volumetric densities, delivery temperatures and pressures, number of cycles, efficiencies, cost, charging and discharging rates, safety, and others. To date, no material or system unequivocally addresses the majority of the different requirements.
Under pressure
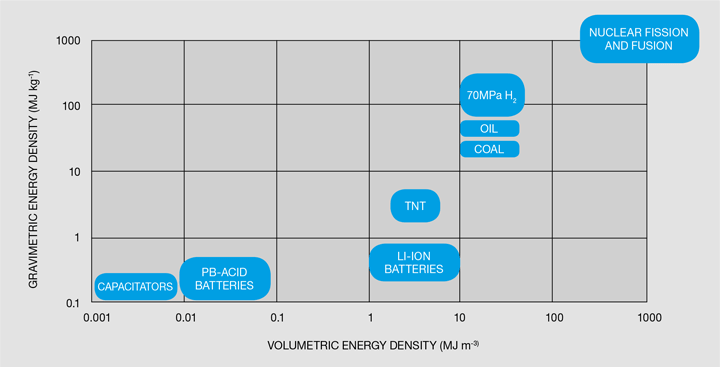
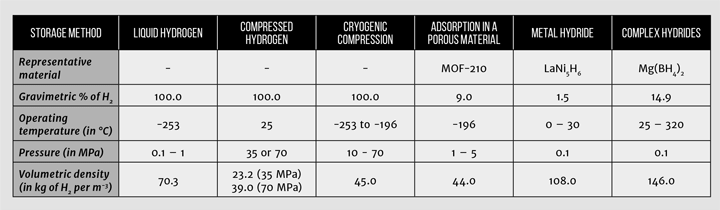
The most conventional way of storing hydrogen and the current state-of-the-art in the chemical industry is to store hydrogen as a gas following compression. In vehicular storage systems, hydrogen is usually stored in compressed hydrogen cylinders at 35 or 70 MPa. For comparison, a typical tyre in a car has a pressure of 0.23 MPa! The hydrogen storage cylinders are classified in four different types: stainless steel or aluminium (type I), fibre-resin composites with thick metallic lining (type II), fibre-resin composites with fully wrapped metallic liners (type III) and polymeric liners fully wrapped in fibre resin composites (type IV), with volumes usually ranging from 0.050–0.200 m3 [5]. Type IV cylinders are the most common option, with a polymeric liner such as high-density polyethylene (HDPE) used as the gas permeation barrier. It is important to remember that hydrogen can easily permeate most materials, which is why properly lining the cylinders is so important. One of the main issues with compression of hydrogen is that it carries a large energy penalty – the energy used in compressing hydrogen to 35 MPa is 14.5 MJ per kg of hydrogen, and if compressed to 70 MPa, then it is 18 MJ per kg of hydrogen [6]. This would mean that, if stored at 70 MPa, about 15% of the energy contained in the hydrogen is spent compressing it! Still, compressed hydrogen is the technology of choice for the chemical industry and for most of the current commercial hydrogen fuel cell vehicles. Compressed hydrogen storage has good volumetric and gravimetric energy densities in comparison with other energy storage methods, as seen in Figure 2.
Cool down
Another conventional storage method to achieve higher volumetric densities is to liquefy hydrogen. The issues associated with liquefaction are that hydrogen’s normal boiling point is –252.9 °C (20.2 K), which is a very difficult temperature to achieve and maintain. As a liquid, hydrogen is typically stored at 20.0 K and with pressures ranging from 0.1–1 MPa. In this state, hydrogen has a volumetric density of 70.3 kg/m3 [6], which compares to volumetric densities of 23.2 kg/m3 and 39.0 kg/m3 for compression at room temperature at 35 and 70 MPa, respectively [1]. Despite the good hydrogen volumetric densities, liquid hydrogen has some issues, at the top of which is boil-off. It is very difficult to completely insulate the storage system, so some hydrogen will heat up and boil off, creating high pressures in the tank, which has to be depressurised by venting the hydrogen. This is an even bigger issue if the tank is left dormant for long periods of time. In addition to boil-off, there is also a large energy penalty with liquefaction of hydrogen, which can be as much as 36 to 47 MJ per kg of hydrogen, which is around 30 to 40% of its lower heating value, making liquefaction of hydrogen a very energy intensive method [6].
How about a mixture of the two?
Due to shortcomings in both liquid and compressed storage, other alternative physical storage methods are being investigated. One of these is cryogenic compression of hydrogen, which is a combination of compression and liquid storage, and tries to mitigate the issues that plague both methods by avoiding high pressures and very low temperatures. The main advantage of cryogenic compression is its flexibility, as it can operate at temperatures as low as 20 K (-253°C) and at pressures up to 25 MPa, with typical operating conditions between 20 and 60 K (-253 °C and -213 °C, respectively). Having hydrogen both compressed and at cryogenic temperatures creates a bigger balance‑of-plant, but hydrogen gravimetric capacities for whole systems can be as high as 45.0 kg/m3 [8]. Much of the work done on cryogenic compression has originated from research in the US Department of Energy laboratories, and some car manufacturers such as BMW are looking into the technology [9].
What about reacting hydrogen with something?
The issues surrounding compressed and liquid storage, especially the ones associated with costs and safety, have led to the development of other storage alternatives. One area that has seen considerable research is chemical storage of hydrogen. In chemical storage, the hydrogen molecule dissociates and reacts with a material, forming a hydride. Most of the issues associated with storing hydrogen as a hydride are related to dehydrogenation, which is difficult as it requires high temperatures, and reversibility, which can be problematic if the storage material is used over many cycles [6, 10]. Hydride materials that have been suggested as hydrogen storage materials include palladium hydride (PdH), magnesium hydride (MgH2) and magnesium borohydride Mg(BH4)2. Hydrogen can also be stored through other routes, including hydrogenation of organic molecules.
The ammonia economy
Another possibility is to take advantage of the current infrastructure available for liquid fuels and use ammonia as a hydrogen carrier. Ammonia has high volumetric and gravimetric hydrogen densities, can be stored as a liquid at moderate pressures and can be decomposed to release hydrogen in a catalytic reaction [11]. The main disadvantages of using ammonia as a hydrogen carrier are related to its toxicity and reversibility, as trace amounts of ammonia are found in the hydrogen after it decomposes [11]. The ammonia needs to be decomposed to release the hydrogen, which is usually done at high temperatures in the presence of a catalyst, with much recent research focussed on low temperature (<300°C) catalytic decomposition of ammonia. In addition to its high densities, ammonia has the advantage of only releasing water and nitrogen when decomposed to release the hydrogen.
Storing hydrogen in nanomaterials
Hydrogen can also be stored through adsorption in a porous material. Adsorption will be very familiar to chemical engineers, as it is widely used in the chemical industry for gaseous separations in adsorption columns. Adsorption of hydrogen is considered an attractive option, as it can offer good densities at moderate pressures and temperatures [6]. In adsorption, the hydrogen molecule does not dissociate and does not react with the substrate material. Hence, hydrogen adsorption typically has fast kinetics and is completely reversible. The operating conditions are milder than other forms, as it is usually done at 77 K and in the 1 to 5 MPa range. Storage of hydrogen through adsorption is highly dependent on the type of material. Ideally, the material has a very high surface areas, as gravimetric hydrogen storage correlates with available surface area, and pores below 1 nm, as these have the highest energy of interaction with the gas [6]. In addition to this, it needs to be low density, low cost, and should possess good mechanical and thermal properties.
Many interesting developments in synthetic chemistry have occurred in the last five years, particularly in the synthesis of highly porous materials with tuneable properties. One excellent example are metal-organic frameworks (MOFs), crystalline materials made from metal centres and organic linkers. Examples of high surface-area metal organic frameworks that have been proposed as hydrogen storage materials include NU-100 [16] and MOF-210 [13], both of which have surface areas in excess of 6,000 m2/g which is almost the area of the Wembley pitch in one gramme of material! Other recently discovered high-surface area materials include porous polymers and novel activated carbons, which can have surface areas in excess of 3,000 m2/g [17, 18]. The different hydrogen storage technologies are presented in Table 1, with gravimetric and volumetric densities, and operating conditions.
With a pinch of salt
In addition to applications in mobile vehicles, hydrogen has also been considered for grid-scale energy storage. In this context, as the storage is usually stationary, gravimetric and volumetric energy densities are less important, and hydrogen is stored underground in salt caverns or depleted oil and gas fields. Underground hydrogen storage is currently being used in the UK. Examples include the Teesside region, where three salt caverns are licensed for operation, each with 70,000 m3. The storage occurs at 4.5 MPa at a depth of 400 m, and the caverns are operated by SABIC, which used it to distribute hydrogen to chemical plants in the area [19].
The storage conundrum
In the transport sector, rather than a competing technology, hydrogen has been considered an excellent complement to battery electric vehicles, since it can be used as a range extender, as batteries start to become uneconomic with large sizes [20]. A hybrid solution that integrates fuel cells with battery systems seems to be the safest bet at the moment for decarbonising the transport sector, except for very small cars in urban areas that have low-cost batteries [20]. In order for hydrogen to be viable as a fuel in mobile applications, appropriate storage systems need to be designed and developed, focussing on affordability, safety and sustainability. While recent studies have shown potential in some technologies, there is still no clear cut answer to the hydrogen storage problem, with industry opting at the moment to use compressed hydrogen cylinders in commercial vehicles, which have safety issues and incur in large energy penalties. There is plenty of room for improvements and innovations in this fascinating topic, and many aspects where chemical engineers can add value. The transport sector is one of the sectors most dependent on fossil fuels, so it is imperative that we find clean and sustainable solutions that can be implemented at a global scale in this sector. Despite all the technical issues still present with hydrogen, there are many advantages of using it as an energy vector, and it is very likely that hydrogen will be part of a global future energy mix.
This is the tenth article in a series discussing the challenges and opportunities of the hydrogen economy, developed in partnership with IChemE’s Clean Energy Special Interest Group. To read more from the series online, visit the series hub.
References
- Leachman, JW, et al, Fundamental Equations of State for Parahydrogen, Normal Hydrogen, and Orthohydrogen. Journal of Physical and Chemical Reference Data, 2009. 38(3).
- Johnston, HL, WE Keller, and AS Friedman, The Compressibility of Liquid Normal Hydrogen from the Boiling Point to the Critical Point at Pressures up to 100-Atmospheres. Journal of the American Chemical Society, 1954. 76(6): p1482-1486.
- Silvera, IF, The Solid Molecular Hydrogens in the Condensed Phase - Fundamentals and Static Properties. Reviews of Modern Physics, 1980. 52(2): p393-452.
- US Department of Energy. DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. Available from www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles. Accessed November 2018.
- Barthelemy, H, Hydrogen storage - Industrial prospectives. International Journal of Hydrogen Energy, 2012. 37(22): p17364-17372.
- Eberle, U, Felderhoff, M and Schuth, F, Chemical and Physical Solutions for Hydrogen Storage. Angewandte Chemie-International Edition, 2009. 48(36): p6608-6630.
- Zuttel, A, et al, Hydrogen: the future energy carrier. Philosophical Transactions of the Royal Society A: Mathematical Physical and Engineering Sciences, 2010. 368(1923): p3329-3342.
- Ahluwalia, RK, et al, Technical assessment of cryo-compressed hydrogen storage tank systems for automotive applications. International Journal of Hydrogen Energy, 2010. 35(9): p4171-4184.
- BMW Group. Press release: First hydrogen station with two types of refuelling technology. Available from www.press.bmwgroup.com. Accessed November 2018.
- Weidenthaler, C and Felderhoff, M, Solid-state hydrogen storage for mobile applications: Quo Vadis? Energy & Environmental Science, 2011. 4(7): p. 2495-2502.
- Klerke, A, et al, Ammonia for hydrogen storage: challenges and opportunities. Journal of Materials Chemistry, 2008. 18(20): p2304-2310.
- Chlopek, K, et al, Synthesis and properties of magnesium tetrahydroborate, Mg(BH4)(2). Journal of Materials Chemistry, 2007. 17(33): p3496-3503.
- Furukawa, H, et al, Ultrahigh Porosity in Metal-Organic Frameworks. Science, 2010. 329(5990): p424-428.
- Orimo, SI, et al, Complex hydrides for hydrogen storage. Chemical Reviews, 2007. 107(10): p4111-4132.
- Sandrock, G, A panoramic overview of hydrogen storage alloys from a gas reaction point of view. Journal of Alloys and Compounds, 1999. 293: p877-888.
- Farha, OK, et al, De novo synthesis of a metal-organic framework material featuring ultrahigh surface area and gas storage capacities. Nature Chemistry, 2010. 2(11): p944-948.
- Strobel, R, et al, Hydrogen storage by carbon materials. Journal of Power Sources, 2006. 159(2): p781-801.
- Svec, F, Germain, J and Frechet, JMJ, Nanoporous Polymers for Hydrogen Storage. Small, 2009. 5(10): p1098-1111.
- Leeds City Gate. H21. Available from www.northerngasnetworks.co.uk/wp-content/uploads/2017/04/H21-Report-Interactive-PDF-July-2016.compressed.pdf. Accessed November 2018.
- Offer, GJ, et al, Techno-economic and behavioural analysis of battery electric, hydrogen fuel cell and hybrid vehicles in a future sustainable road transport system in the UK. Energy Policy, 2011. 39(4): p1939-1950.
Recent Editions
Catch up on the latest news, views and jobs from The Chemical Engineer. Below are the four latest issues. View a wider selection of the archive from within the Magazine section of this site.